american academy of pediatrics covid vaccine under 12
Ad Maximize protection get all recommended vaccine doses and boosters as soon as possible. Food and Drug Administration FDA to approve the use of COVID-19 vaccines in children younger than age 12.
/kidcovid-514a2faa59744c7f8e7de7a8cbba640d.jpg)
How Does The Delta Variant Affect Kids
Neither the Johnson Johnson or Moderna vaccine have been approved for anyone under age 18.
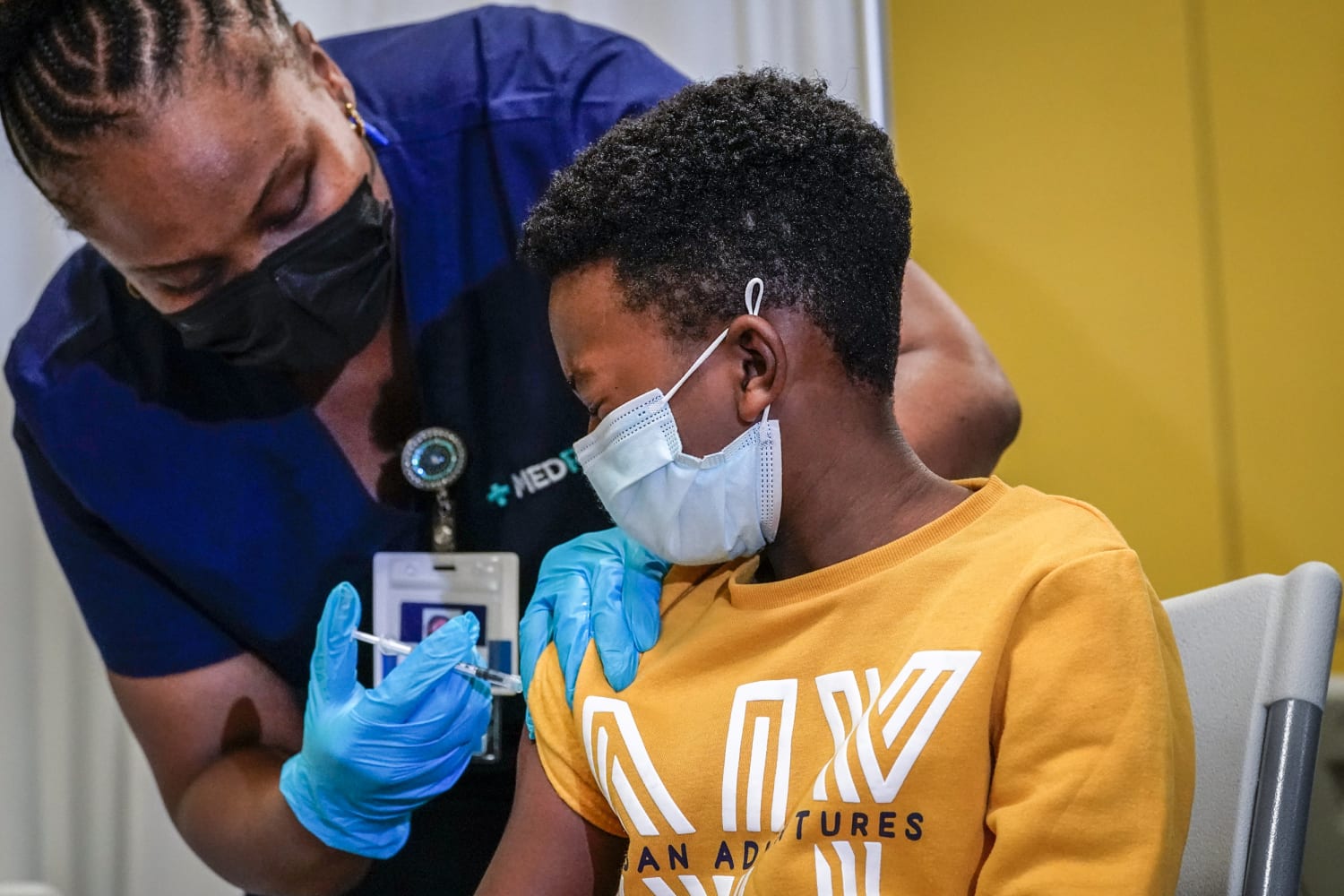
. COVID-19 Vaccine Campaign Toolkit. The vaccine will continue. The American Academy of Pediatrics recommends COVID-19 vaccines for all eligible children 5 years and older.
At a time when more than 75 percent of US. But there are still important reasons to get itheres everything you need to know. The American Academy of Pediatrics AAP is an organization of 67000 primary care pediatricians pediatric medical subspecialists and pediatric surgical specialists dedicated to the health safety and well-being of all infants children adolescents and young adults.
The Centers for Disease Control and Prevention CDC director signed off on boosters for this group Wednesday after an advisory group voted 13-1 in favor and called for a strong recommendation. The American Academy of Pediatrics AAP in collaboration with the Centers for Disease Control and Prevention CDC conducted 9 key informant interviews with emerging pediatric practices to identify innovative strategies to continue conducting developmental surveillance screening referral and follow-up during the COVID-19 pandemic. American Academy of Pediatrics Cautions Against Off-Label Use of COVID-19 Vaccines in Children Under 12.
COVID-19 Vaccine Campaign Toolkit. Vaccination protects your child from becoming seriou. The American Academy of Pediatrics AAP recommends the following related to coronavirus disease 2019 COVID-19 vaccine in children and adolescents.
Download the Full Report 3302022. On May 12 2021 CDC approved the use of the Pfizer-BioNTech COVID-19 vaccine in persons aged 12-15 years following the vaccines EUA granted by the FDA on May 10th. The American Academy of Pediatrics AAP is urging the US.
The American Academy of Pediatrics recommends COVID-19 vaccines for all eligible children 5 years and olderMillions of kids and teens already have been safely vaccinatedVaccination protects your child from becoming seriously ill or dying from COVID. Adults have received at least one vaccine dose and just about everything has opened up the American Academy of Pediatrics reported over 250000 new. The COVID-19 vaccine is less effective at preventing infection in kids under 12.
While questions may now arise about the administration of the vaccine off-label for children aged 11. Currently only the Pfizer COVID-19 vaccine has an emergency use authorization for children 12 and up. ITASCA IL -- Today the Food and Drug Administration FDA finalized the full approval of the Pfizer-BioNTech COVID-19 vaccine for ages 16 and older.
The American Academy of Pediatrics AAP is an organization of 67000 primary care pediatricians pediatric medical subspecialists and pediatric surgical specialists dedicated to the health safety and well-being of all infants children adolescents and young adults. The American Academy of Pediatrics said the rise of the Delta variant changes the risk-benefit analysis for authorizing vaccines in children Skip to Article Set weather. The best way to slow the emergence of COVID variants is to get vaccinated.
The vaccine was found to be 907 effective in children ages 5-11 and 100 effective in children ages 12- 15The COVID-19. The FDA issued an EUA for the Pfizer vaccine for kids aged 5 to 11 on 10292021 followed by ACIP recommendation and CDC approval on 1122021. The AAP recommends COVID-19 vaccination for all children and adolescents 5 years of age and older who do not have contraindications using a COVID-19 vaccine authorized for use for their age.
Adolescents ages 12-15 years can begin receiving COVID-19 vaccine boosters. The dose may be different for younger ages. As Covid cases among children continue to rise in the US due to spread of the Delta variant experts are urging the federal government to fast-track vaccine approval for those under the age of 12.
The AAP recommends against giving the vaccine to children under 12 years until authorized by the FDA The FDAs licensure of the vaccine which will be called Comirnaty applies to ages 16 years and older the group for whom emergency use authorization EUA was granted in December 2020. The AAP recommends against giving the vaccine to children under 12 until authorized by the FDA While the FDA is considering full approval of the vaccine for ages 12 through 15 it is available under emergency use authorization for this age group now and AAP strongly recommends all eligible adolescents be vaccinated as soon as possible. AAP CDC recommend COVID-19 vaccine for ages 12 and older May 12 2021 In addition to approving the Pfizer-BioNTech vaccine for adolescents the CDC updated its clinical guidance to allow coadministration of vaccines.
MRNA COVID-19 vaccines are FDA-approved or authorized for a 3-week Pfizer-BioNTech vaccine or 4-week Moderna vaccine interval between the first and second dose. The Food and Drug Administration must work aggressively toward authorizing a Covid-19 vaccine for children under age 12 the head of the American Academy of Pediatrics wrote in a letter to Dr. A 3- or 4-week interval continues to be the recommended interval for people who are moderately to severely immunocompromised children who are 5-11 years old and others who need rapid protection.
NPRs Ailsa Chang speaks with American Academy of Pediatrics President Lee Savio Beers about the mounting pressure to consider emergency use authorization of COVID-19 vaccines for children under 12. COVID-19 Vaccine Campaign Toolkit. The American Academy of Pediatrics AAP is an organization of 67000 primary care pediatricians pediatric medical subspecialists and pediatric surgical specialists dedicated to the health safety and well-being of all infants children adolescents and young adults.
American Academy of Pediatrics urges FDA to approve COVID vaccines for children under 12 Sep 13 2021 640 PM EDT. Millions of kids and teens already have been safely vaccinated. FDA authorizes COVID-19 vaccine for adolescents ages 12-15.

Us Officials Set Stage For Vaccination Campaign For Younger Children Coronavirus The Guardian
Aap Recommends All Children 12 And Older Get The Covid 19 Vaccine
Are Kids At Higher Risk For Re Infection Of Covid 19

Nearly 1 Million Kids Under Age 12 Have Had The Covid Vaccine Wh Says
600 000 Children Ages 12 15 Have Received Their First Covid 19 Vaccine Dose

As Omicron Surges Effort To Vaccinate Young Children Stalls Kaiser Health News

Kids Covid Vaccine Twitter Chat Twitter

Q A Aap Endorses Giving Routine Immunizations Covid 19 Vaccines At Same Time
American Academy Of Pediatrics Urges Fda To Approve Covid Vaccines For Children Under 12 Youtube

Some Parents Push To Give Covid 19 Vaccine To Children Under 12 Against Government Guidance
Covid Vaccines For Kids Under 12 Expected Midwinter Fda Official Says

Kids Under 12 Could Be Eligible For A Vaccine Against Covid This Fall Shots Health News Npr
More Children Are Catching Covid 19 But It S Unclear If They Re Getting Sicker Coronavirus Updates Npr

American Parents Lie To Get Covid Vaccine For Under 12s News The Times
What Pediatricians Want Parents To Know About The Covid Vaccine For Kids
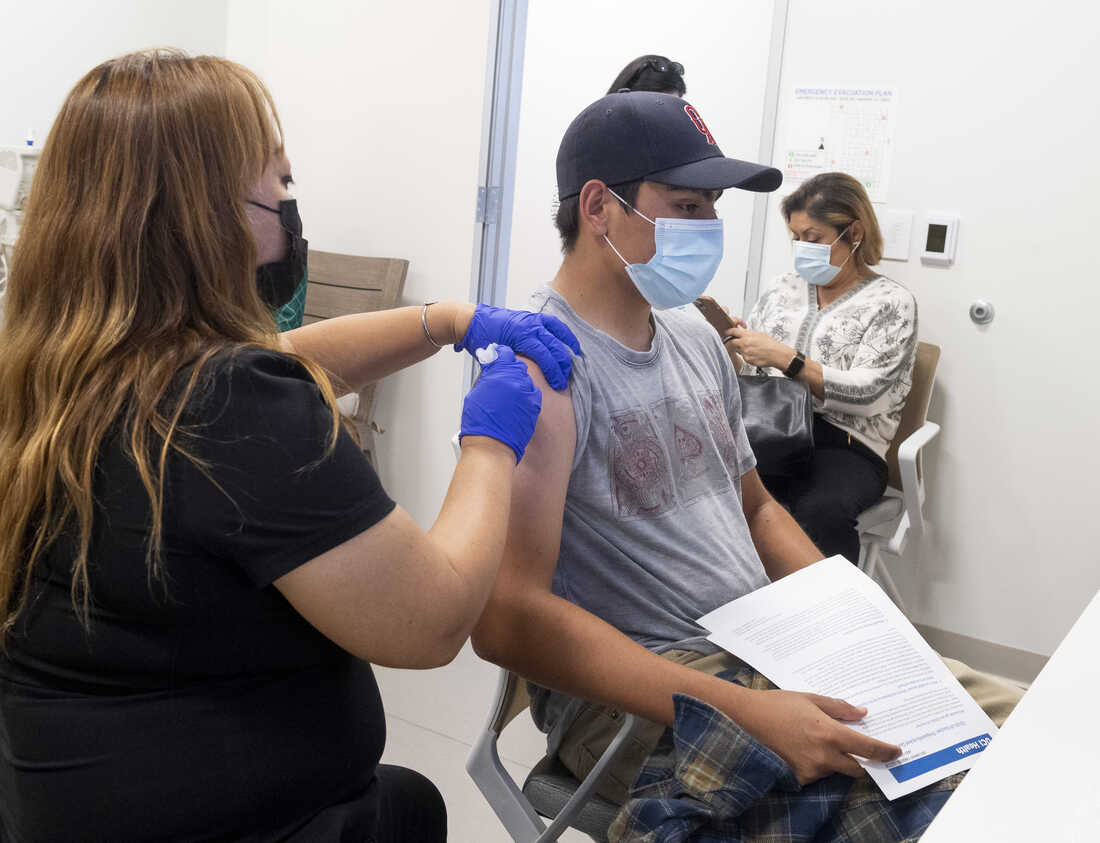
Faq About Pfizer S Covid Vaccine And Kids Shots Health News Npr

Covid And Kids Coronavirus Cases In Children Increased By Nearly 240 Since July American Academy Of Pediatrics Says Abc7 Chicago

Fact Check How Useful Are Coronavirus Vaccines For Children And Adolescents Coronavirus And Covid 19 Latest News About Covid 19 Dw 06 08 2021
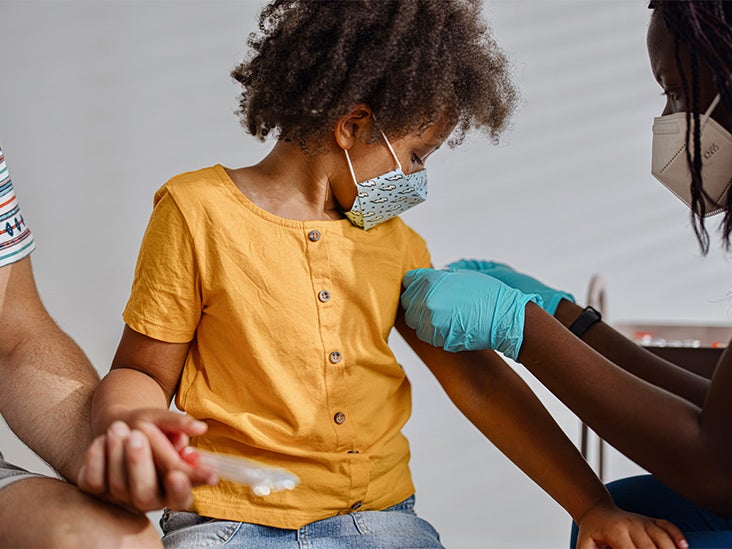
What Parents Should Know About Covid 19 Vaccines For Kids Under 12